Technology
Hydrogen on demand
We are pioneering an approach to produce hydrogen on demand, eliminate the problems of efficient hydrogen storage and transportation.
The problem
Key obstacles preventing green hydrogen from becoming a mainstream energy source relate to its storage and transportation.
Hydrogen is a highly reactive gas- it can penetrate and leak through almost any material deteriorating storage equipment and shortening its useful lifespan.
Hydrogen also has low volumetric energy density, meaning the density of the stored hydrogen is significantly less than the density of ordinary fossil fuels. A 40-ton truck can deliver 26 tons of liquefied petrol gas. The same truck can deliver just 360 kg of compressed hydrogen, which is 72 times less by mass than ordinary gas.
These factors make transporting and storing hydrogen a challenge. Current solutions of cooling, compressing or binding hydrogen to ammonia, are energy-demanding, expensive, require specialised infrastructure and pose environmental risk.
Our solution
We are able to extract green hydrogen on-site and on-demand, sidestepping all transportation and storage challenges typically associated with hydrogen.
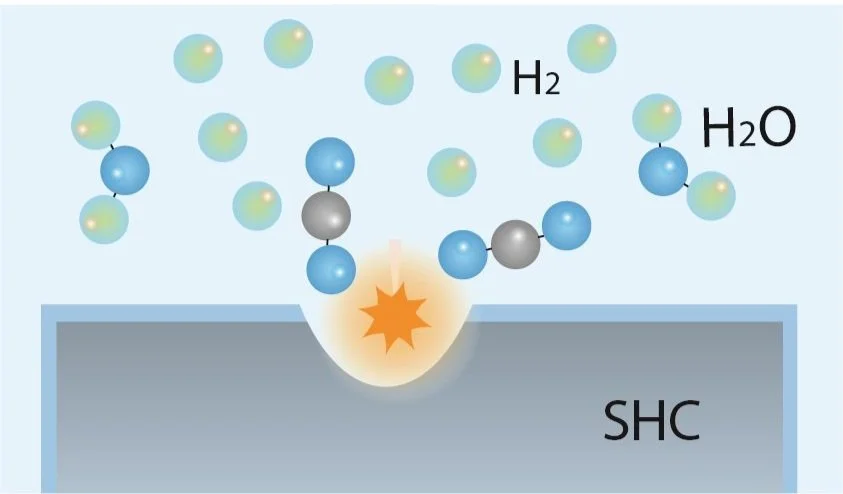
Similar to electrolysis, our approach extracts hydrogen from water and other suitable liquids. Using the latest technology, we create a reaction between water and certain solid input materials - which we are calling Solid Hydrogen Cells (SHC).
Specifically, we generate a substitution reaction between water and the material - a by-product of this reaction is a controlled release of pure hydrogen.
Schema of the process
The input material, or SHC, plays a double role in the process: as a chemical agent that extracts hydrogen from water through substitution reactions and as a compact chemical storage allowing us to effectively deliver hydrogen.
SHCs are inert at standard conditions, safe to store for years and transport across large distances. The compound can be produced from widely available components sourced from waste, mining or steel-production industries.
When required, SHCs produce hydrogen by reacting with water under specific conditions.
Example of SHC used in testing
This approach does not require pure water and can work with any kind of naturally available water. We also avoid the use of any acids or alkali bases that are commonly used in electrolysis, and instead, we need just tiny amounts of catalyst.
Benefits
Expected benefits of this technology include:
On-site and on-demand hydrogen production.
Zero carbon emission.
Very rapid rate of hydrogen generation.
High purity of the produced hydrogen, ready to be used directly in fuel cells.
Hydrogen production by portable stations on-site using any kind of water including seawater.
Minimal labour and power to operate machinery.
Low logistical, operational, and storage costs.
By our estimates, the proposed approach carries significant cost saving for storage and transportation as the final cost of hydrogen produced on-site. This technology also paves the way for portable or stationary hydrogen plants to be established in places where the cost of traditional energy producing plants is not justified.